Printable Periodic Table Electronegativity Solution A Electronegativity increases from lower left to upper right in the periodic table Figure 2 12 2 Because Sr lies far to the left of the other elements given we can predict that it will have the lowest electronegativity Because Cl lies above and to the right of Se we can predict that Cl Se
You will find the atomic number the atomic mass mean relative the symbol of course melting point C boiling point C electronegativity density and a radioactivity flag in them The advanced layout also provides electron configuration as well as oxidation states Groups are numbered following the newest IUPAC recommendation Printable Image SVG in browser Periodic Table with Element Names and Electronegativity This periodic table chart lists elements by name in alphabetical order including the element symbol atomic number and Pauling electronegativity value for quick and simple reference
Printable Periodic Table Electronegativity
/PeriodicTableEnegativity-56a12c955f9b58b7d0bcc69d.png) Printable Periodic Table Electronegativity
Printable Periodic Table Electronegativity
https://fthmb.tqn.com/QE8E2ZHB2E-USqzv5flMhxT19k0=/2200x1701/filters:fill(auto,1)/PeriodicTableEnegativity-56a12c955f9b58b7d0bcc69d.png
Electronegativity is a measure of the ability of an atom to attract the electrons when the atom is part of a compound Electronegativity values generally increase from left to right across the periodic table Electronegativities generally decrease from the top to bottom of a group The highest electronegativity value is for fluorine
Pre-crafted templates provide a time-saving solution for producing a diverse variety of documents and files. These pre-designed formats and designs can be utilized for various personal and expert tasks, including resumes, invites, flyers, newsletters, reports, presentations, and more, improving the content development procedure.
Printable Periodic Table Electronegativity
.PNG)
Periodic Trends Presentation Chemistry
.PNG)
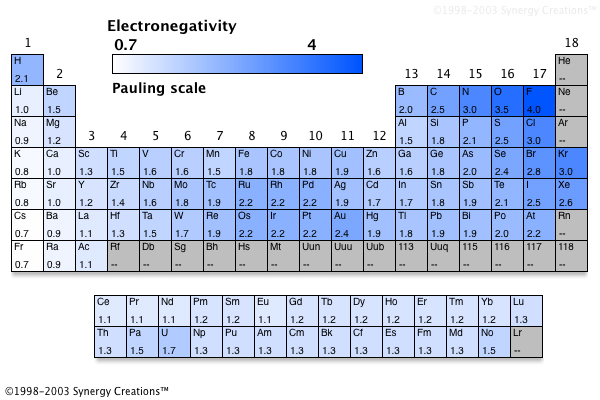
Periodic Table Of Electronegativities
Which Element In The Periodic Table Has The Greatest Electronegativity

Complete Periodic Table Of Elements Pdf Elcho Table
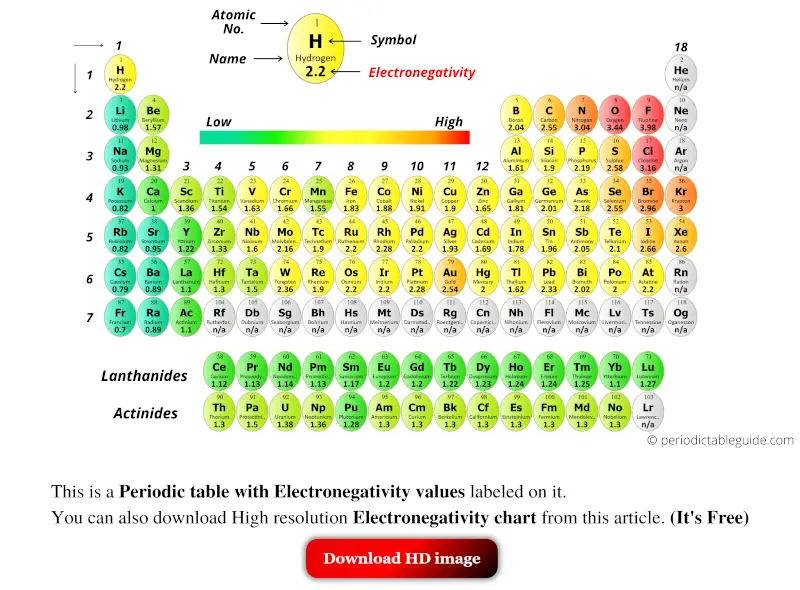
Periodic Table With Electronegativity Values Labeled Image
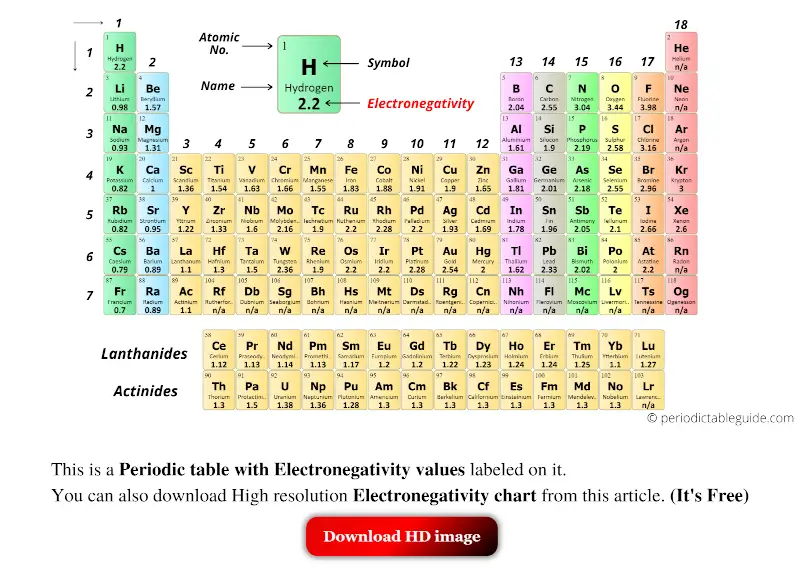
Periodic Table With Electronegativity Values Labeled Image
/PeriodicTableEnegativity-56a12c955f9b58b7d0bcc69d.png?w=186)
https://www.sciencegeek.net/tables/Electronegativity.pdf
The Periodic Table of the Elements with Electronegativities 1 18 Hydrogen 1 H 1 01 2 1 2 Alkali metals Alkaline earth metals Transition metals Lanthanides Actinides Other metals Metalloids semi metal Nonmetals 6 94 Halogens Noble gases Element name 80 Symbol Beryllium Electronegativity Mercury Hg 200 59 1 9 Atomic Lithium Avg Mass 13 14

https://www.thoughtco.com/printable-periodic-table-electronegativity
This color printable periodic table contains the element number element symbol and electronegativity The colors differentiate between different electronegativity ranges

https://sciencenotes.org/printable-periodic-table
This free periodic table is color coded to indicate the electronegativity of an atom of an element Electronegativity is a trend naturally exhibited in the periodic table but as you can see it s not a hard and fast trend

https://www.thoughtco.com/printable-periodic-tables-4064198
Printable Periodic Tables This periodic table lists the electronegativities of the elements Todd Helmenstine You can download and print the pdf file of the printable periodic table of the elements This periodic table gives the electronegativity value for each of the elements

https://pubchem.ncbi.nlm.nih.gov/periodic-table/electronegativity
Periodic Table Electronegativity in the Periodic Table of Elements Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons Choose Elements to Display Plot of Electronegativity Pauling Scale vs Atomic Number 1 3 11 19 37 55 87 118 Atomic Number 0 1 2 3 4 Electronegativity Pauling Scale
116 Lv Livermorium 293 117 Ts Tennessine 294 118 Og Oganesson 294 For elements with no stable isotopes the mass number of the isotope with the longest half life is in parentheses Interactive periodic table showing names electrons and oxidation states Electronegativity 1 H hydrogen 2 300 2 He helium 4 160 3 Li lithium 0 912 4 Be beryllium 1 576 5 B boron 2 051 6 C carbon 2 544 7 N nitrogen 3 066 8 O oxygen 3 610 9 F fluorine 4 193 10 Ne neon 4 787 11 Na sodium 0 869 12 Mg magnesium 1 293 13 Al aluminium 1 613 14 Si silicon 1 916 15 P phosphorus 2
Patterns of electronegativity in the Periodic Table Note Trends in electronegativity across a period Trends in electronegativity down a group Explaining the patterns in electronegativity Why does electronegativity increase across a period Why does electronegativity fall as you go down a group Diagonal relationships in the