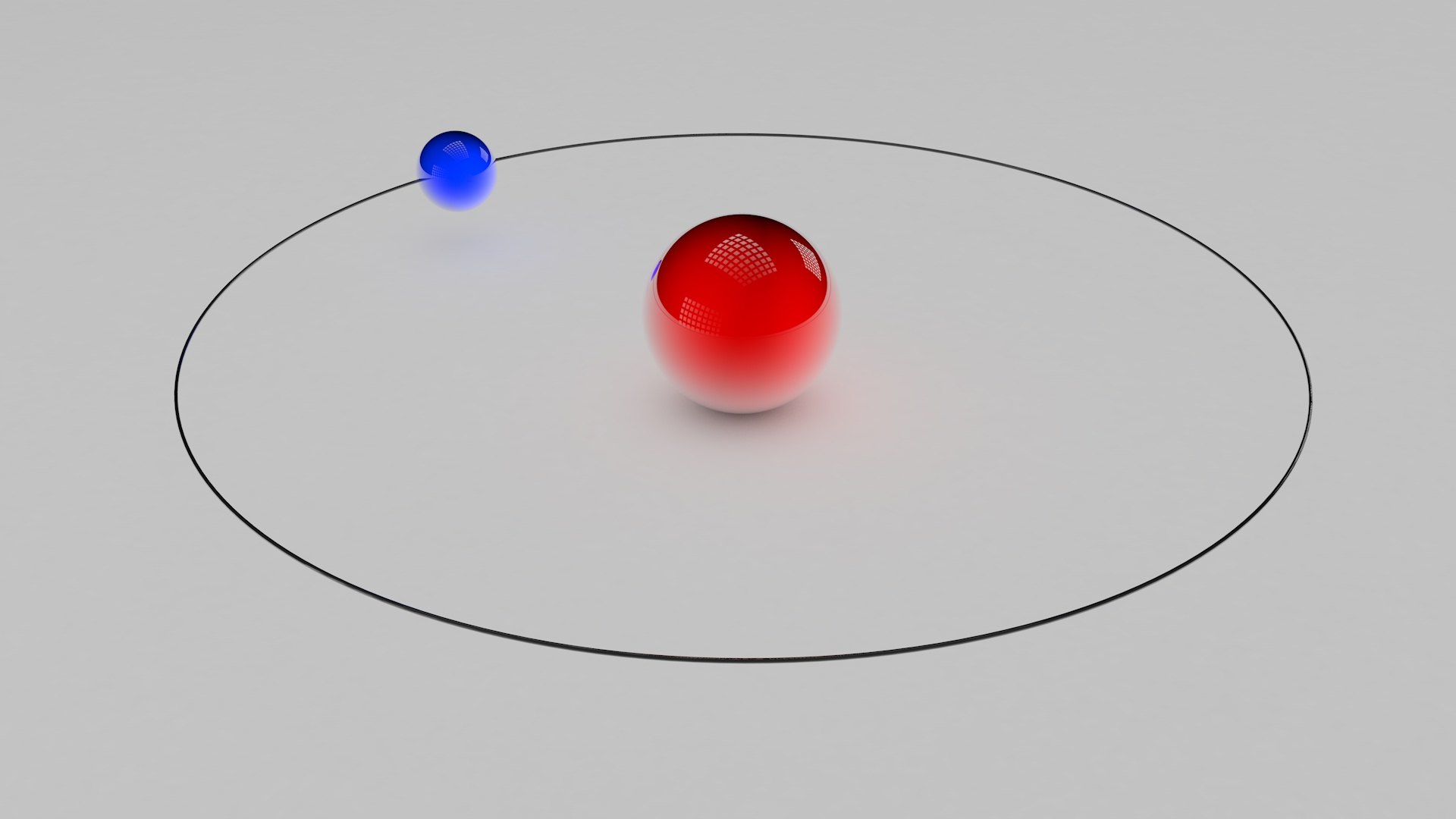
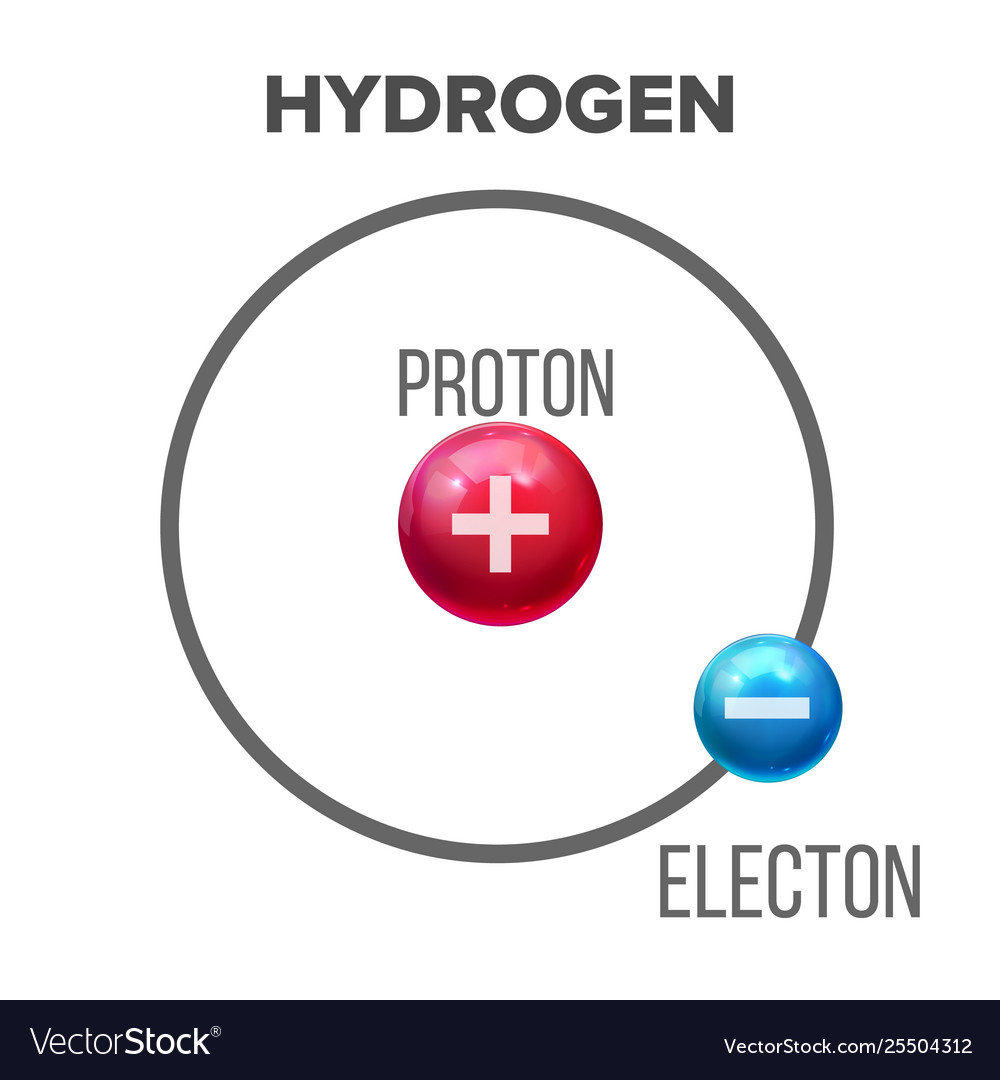
Printable Atomic Model Hydrogen Bohr s theory explained the atomic spectrum of hydrogen and established new and broadly applicable principles in quantum mechanics Figure 30 13 Niels Bohr Danish physicist used the planetary model of the atom to explain the atomic spectrum and size of
Bohr s model of the hydrogen atom proposed by Niels Bohr in 1913 was the first quantum model that correctly explained the hydrogen emission spectrum Bohr s model combines the classical mechanics of planetary motion with the quantum concept of Bohr s theory explained the atomic spectrum of hydrogen and established new and broadly applicable principles in quantum mechanics Figure PageIndex 1 Niels Bohr Danish physicist used the planetary model of the atom to explain the atomic spectrum and size of the hydrogen atom
Printable Atomic Model Hydrogen
 Printable Atomic Model Hydrogen
Printable Atomic Model Hydrogen
https://cdn3.vectorstock.com/i/1000x1000/43/12/bohr-model-of-scientific-hydrogen-atom-vector-25504312.jpg
The Bohr model can be readily extended to hydrogenlike ions systems in which a single electron orbits a nucleus of arbitrary atomic number Z Thus Z 1 for hydrogen Z 2 for mathsf He Z 3 for mathsf Li and so on The Coulomb potential ref 5 generalizes to V r dfrac Ze 2 r label 18
Pre-crafted templates provide a time-saving service for developing a diverse range of files and files. These pre-designed formats and designs can be made use of for different individual and professional tasks, consisting of resumes, invitations, leaflets, newsletters, reports, discussions, and more, streamlining the content creation process.
Printable Atomic Model Hydrogen
The Best 28 Downloadable Atomic Habits Worksheets Pdf Tenverulicah

Pin By Valentina Guarin P On Studying Math Chemistry Lessons Physics

30 Atomic Habits Worksheets Pdf

Free Printable Atomic Habits Worksheets Pdf Printable Templates
3D Model Atom Hydrogen TurboSquid 1616190
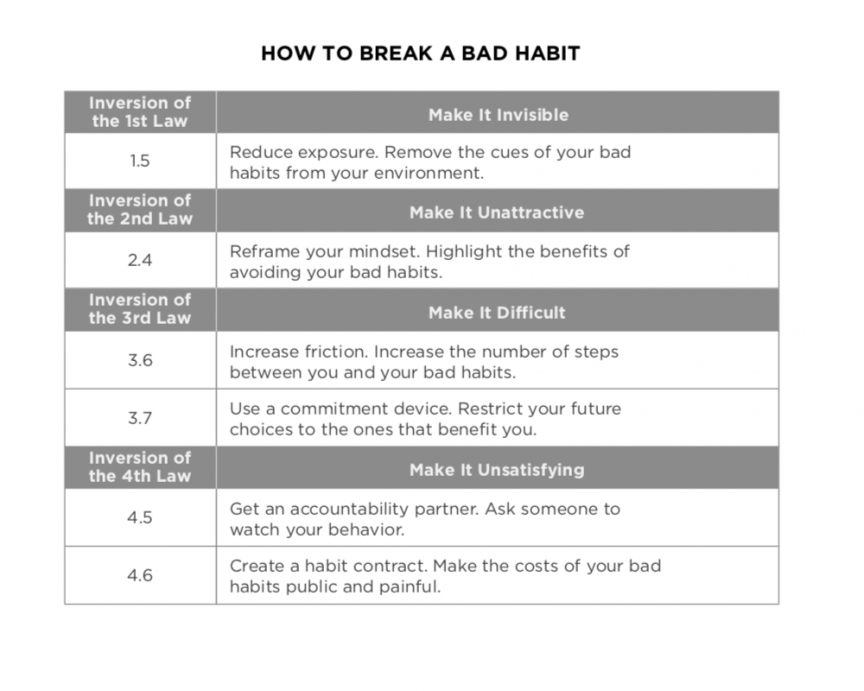
Free Printable Atomic Habits Cheat Sheet Printable Templates
https://chem.libretexts.org//09._The_Hydrogen_Atom/Bohr's_Hydroge…
Niels Bohr introduced the atomic Hydrogen model in 1913 He described it as a positively charged nucleus comprised of protons and neutrons surrounded by a negatively charged electron cloud In the model electrons orbit the nucleus in atomic shells

https://phet.colorado.edu/en/simulation/hydrogen-atom
Try out different models by shooting light at the atom Check how the prediction of the model matches the experimental results

https://bohr.physics.berkeley.edu/classes/221/notes/hydrogen.pdf
Hydrogen is also a basic model for the physics of atoms providing a context and a reference for all of atomic physics and as well as for the physics of collections of atoms including molecules and most systems of interest in condensed matter physics

https://openstax.org//pages/6-4-bohrs-model-of-the-hydrogen-atom
Bohr s model of the hydrogen atom proposed by Niels Bohr in 1913 was the first quantum model that correctly explained the hydrogen emission spectrum Bohr s model combines the classical mechanics of planetary motion with the quantum concept of photons

https://phys.libretexts.org/Bookshelves/University_Physics/Book
Learning Objectives By the end of this section you will be able to Describe the hydrogen atom in terms of wave function probability density total energy and orbital angular momentum Identify the physical significance of each of the quantum numbers n l m of the hydrogen atom
Hydrogen is the chemical element with the symbol H and atomic number 1 Hydrogen is the lightest element At standard conditions hydrogen is a gas of diatomic molecules having the formula H 2 It is colorless odorless tasteless non toxic and highly combustible Hydrogen Element information properties and uses Periodic Table Element Hydrogen H Group 1 Atomic Number 1 s block Mass 1 008 Sources facts uses scarcity SRI podcasts alchemical symbols videos and images Jump to main content Periodic Table Home History Alchemy Podcast Video Trends Periodic Table Home
In 1885 a Swiss mathematics teacher Johann Balmer 1825 1898 showed that the frequencies of the lines observed in the visible region of the spectrum of hydrogen fit a simple equation that can be expressed as follows constant 1 22 1 n2 7 3 1 7 3 1 c o n s t a n t 1 2 2 1 n 2 where n 3 4 5 6