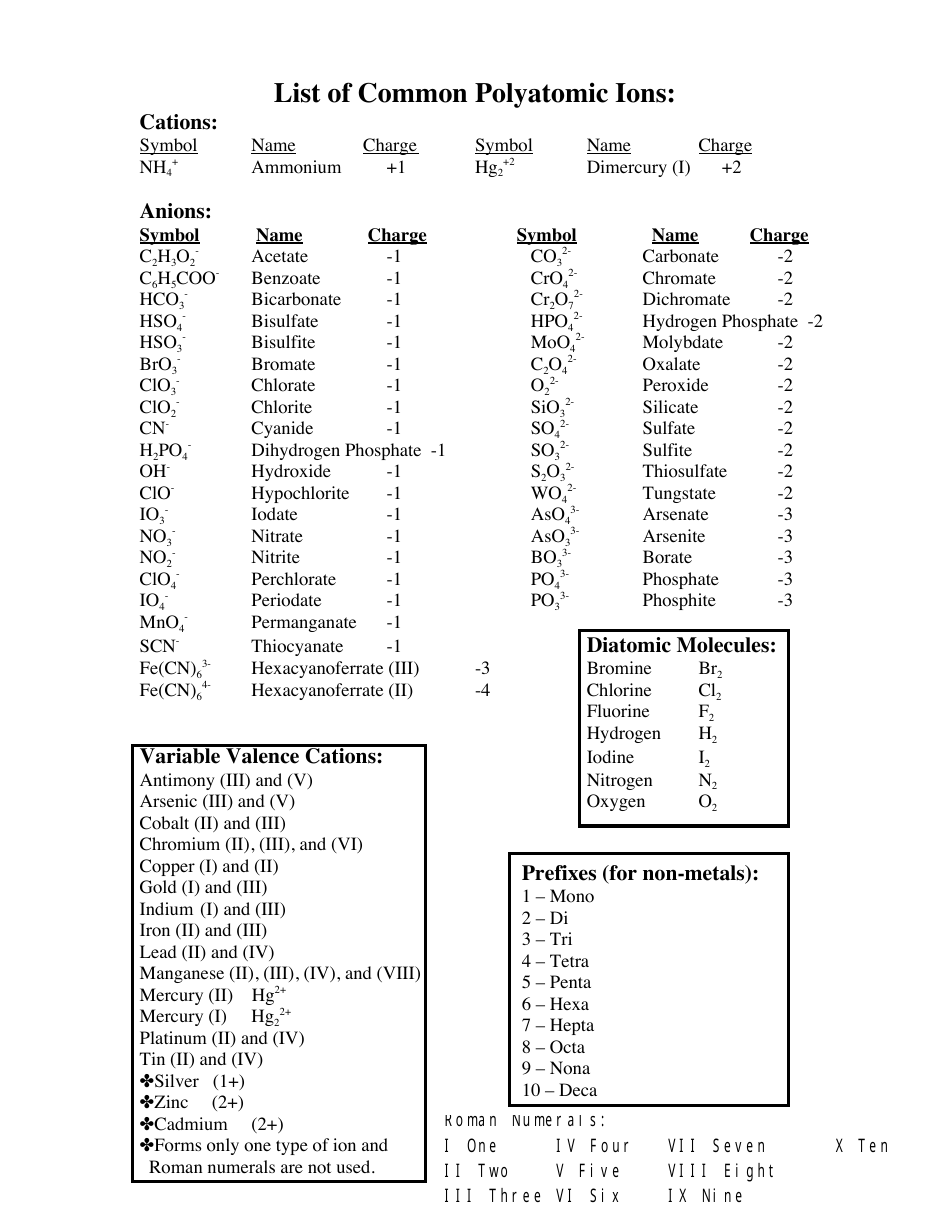
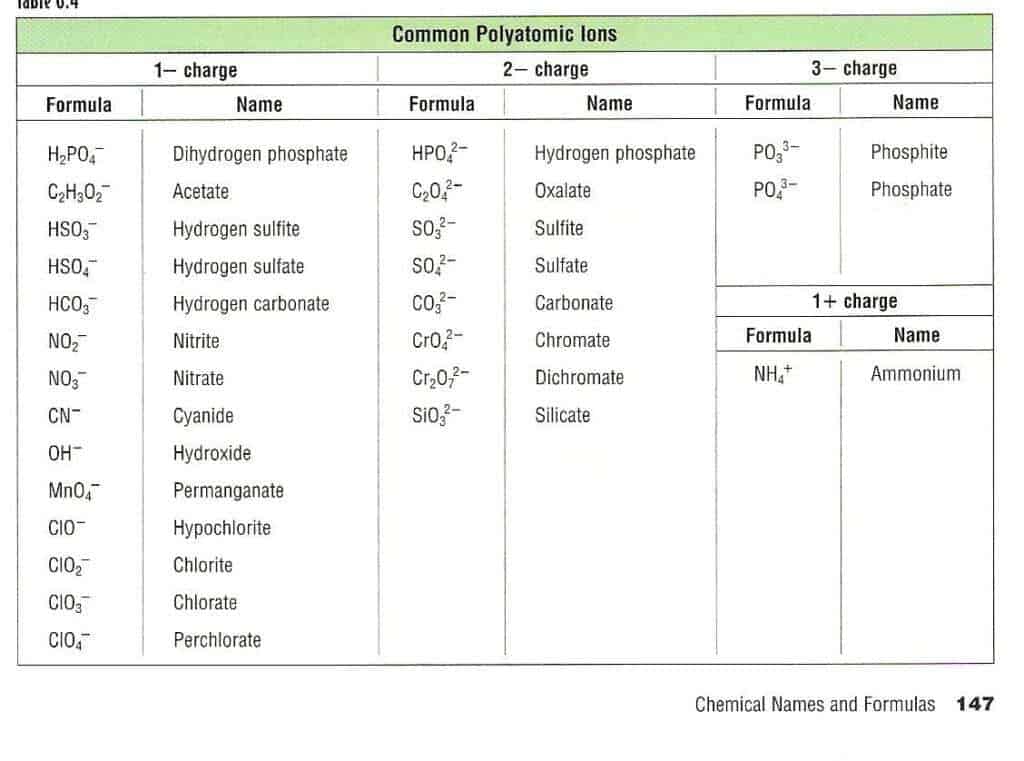
Polyatomic Ion Chart Printable The structures names and formulas of some polyatomic ions are found in Table 3 3 1 Table 6 3 1 Some Polyatomic Ions Polyatomic ions have defined formulas names and charges that cannot be modified in any way Table 6 3 2 lists the ion names and ion formulas of the most common polyatomic ions
Common Polyatomic Ions Most commonly encountered ions in bold Polyatomic Ions A group of atoms held together by covalent bonds found in ionic compounds Know memorize recognize names formulas and charges Polyatomic ions have defined formulas names and charges that cannot be modified in any way Table PageIndex 1 lists the ion names and ion formulas of the most common polyatomic ions For example ce NO 3 is the nitrate ion it has one nitrogen atom and three oxygen atoms and an overall 1 charge
Polyatomic Ion Chart Printable
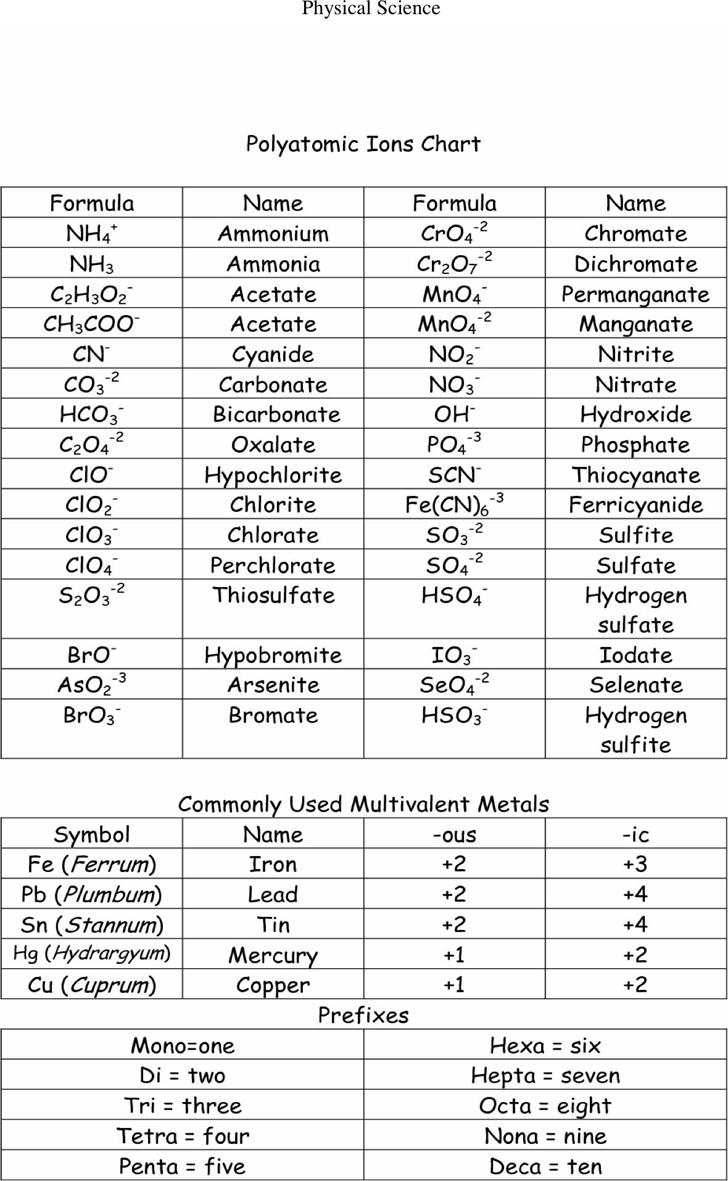 Polyatomic Ion Chart Printable
Polyatomic Ion Chart Printable
https://www.speedytemplate.com/polyatomic-ions-chart-3-1.png
Formulas for ionic compounds contain the symbols and number of each atom and or polyatomic ion present in a compound in the lowest whole number ratio The process of naming ionic compounds with polyatomic ions are the same as
Templates are pre-designed files or files that can be utilized for different functions. They can conserve effort and time by supplying a ready-made format and layout for producing various type of material. Templates can be used for personal or expert jobs, such as resumes, invitations, flyers, newsletters, reports, presentations, and more.
Polyatomic Ion Chart Printable
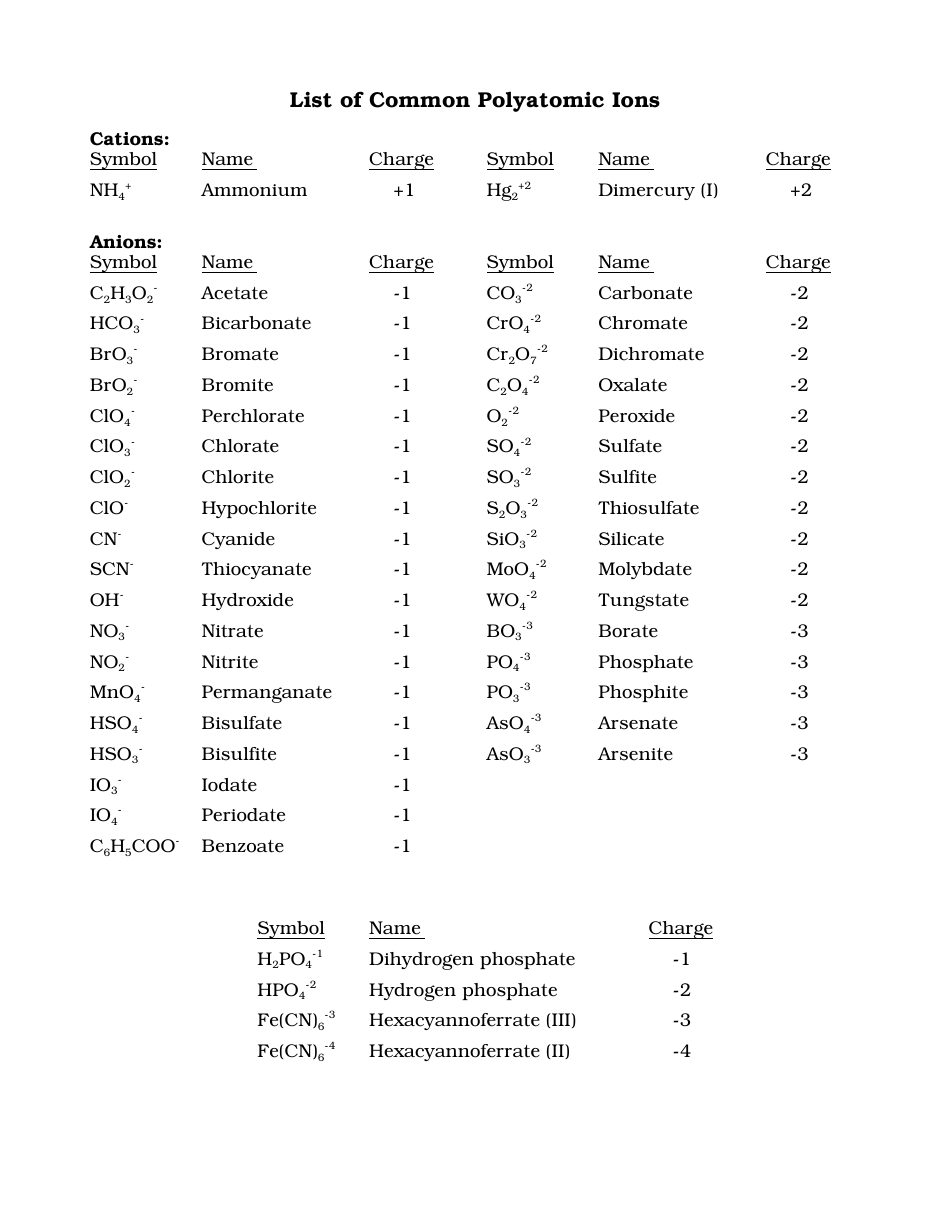
Acid Naming Guidelines ChemTalk TechTrendsClub

5 Polyatomic Ion Charts Word Excel Templates

5 Polyatomic Ion Charts Word Excel Templates
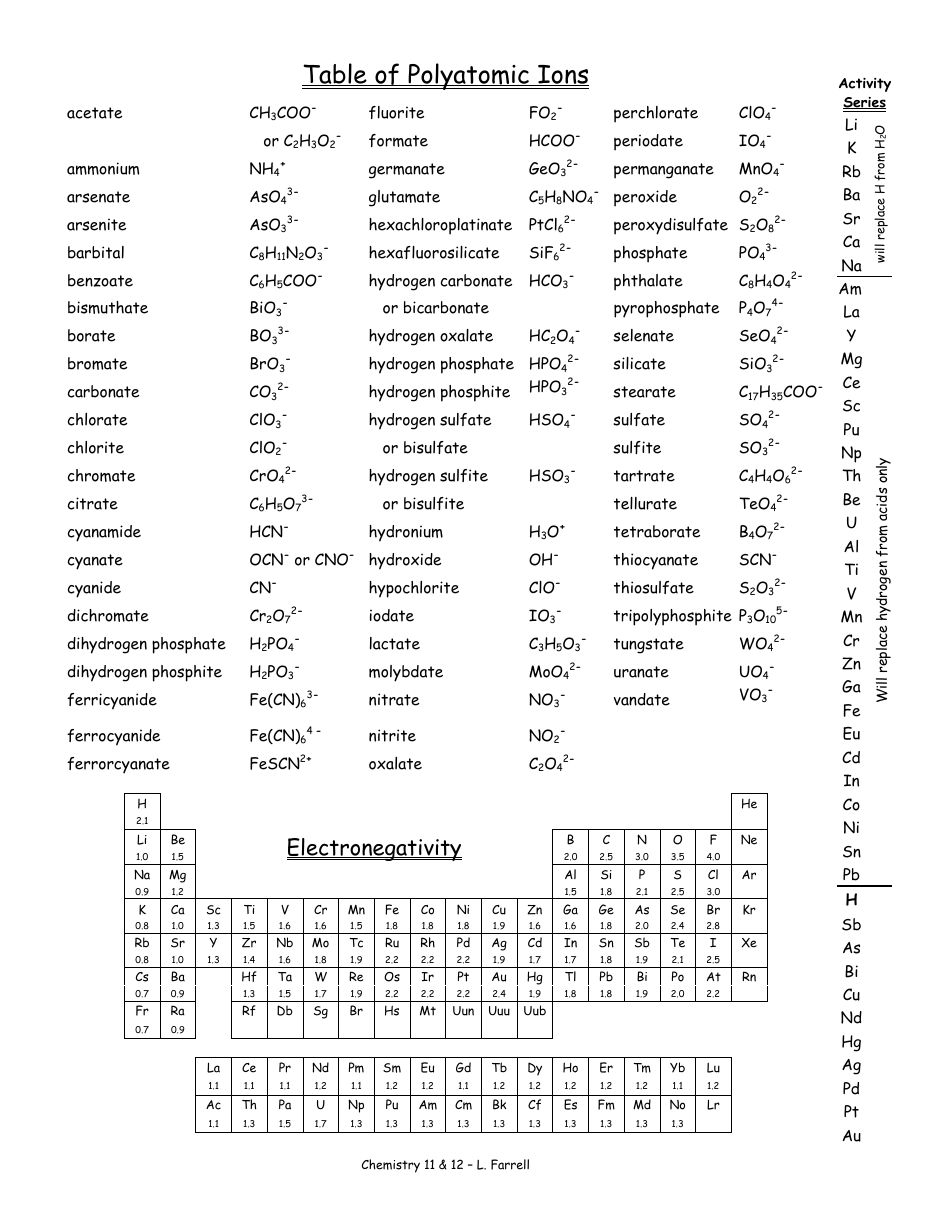
Polyatomic Ions Worksheet Answers

22 Table Of Polyatomic Ions SimonneElwood
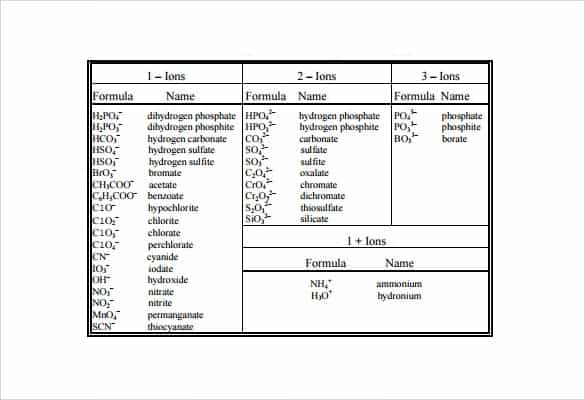
5 Free Polyatomic Ion Charts Word Excel Fomats

https://teachers.wrdsb.ca/gmarlow/files/2019/09/2DI-PERIODIC …
Common Polyatomic ions Ammonium 1 NH4 Hydroxide OH 1 Nitrate 1 2 NO3 Sulfate SO4 1 2 Nitrite NO2 Sulfite SO3 Chlorate ClO3 1 Carbonate CO3 2 Cyanide CN 1 3 Phosphate PO4 Hydrogen sulfate HSO4 1 3 Phosphite PO3 Hydrogen carbonate HCO3 1 2 Peroxide O2 bicarbonate Hydrogen phosphate HPO4 2
https://ch301.cm.utexas.edu/help/ch301/polyatomics.pdf
Common Polyatomic Ions Name s Formula Name s Formula ammonium NH4 acetate CH3COO C2H3O2 bromate BrO3 carbonate CO3 2 chlorate ClO3 chlorite ClO2 chromate CrO4 2 cyanide CN dichromate Cr2O7 2 hydrogen carbonate bicarbonate HCO3 hydrogen sulfate bisulfate HSO4 hydrogen phosphate biphosphate HPO4 2 hydroxide OH

https://www.sciencegeek.net/tables/PT_ions.pdf
PERIODIC TABLE OF IONS acetate CH3COO TABLE OF POLYATOMIC IONS oxalate C2O42 arsenate AsO43 dihydrogen phosphate H2PO4 perchlorate ClO4 arsenite AsO33 hydrogen carbonate HCO3 periodate IO4 benzoate C6H5COO hydrogen oxalate HC2O4 permanganate MnO4 borate BO33 hydrogen sulfate HSO4
https://www.khanacademy.org//a/polyatomic-ions
In this article we will discuss polyatomic ions The prefix poly means many so a polyatomic ion is an ion that contains more than one atom This differentiates polyatomic ions from monatomic ions which contain only one atom Examples of monatomic ions include Na Fe 3 Cl and many many others This article assumes

http://kleinbnhs.weebly.com//2/8/0/2/28026625/ibn_polyatomi…
POLYATOMIC IONS CHART NAME 1 Charge 2 Charge 3 Charge Aluminate AlO 2 Tetraborate B 4 O 7 2 Arsenite AsO3 3 Metaborate BO
Polyatomic ions carrying negative charge Hydroxide OH Acetate CH 3 COO Cyanide CN Chlorate ClO 3 Chlorite ClO 2 Nitrate NO 3 Nitrite NO 2 Hypochlorite OCl Hypobromite OBr Iodate IO 3 Bisulphite HSO 3 Bisulphate HSO 4 Permanganate MnO 4 Thiocyanate SCN Hydrogen carbonate HCO 3 Polyatomic ions are molecular ions composed of two or more atoms bonded by covalent bonds and acting as a single unit but unlike molecules they have a net charge on them The examples include cations like ammonium ion ce NH4 and hydronium ion ce H3O and anions like hydroxide ion ce OH and cyanide ion ce CN
Polyatomic Ions A polyatomic ion is an ion composed of more than one atom The ammonium ion consists of one nitrogen atom and four hydrogen atoms Together they comprise a single ion with a 1 charge and a formula of NH 4