Chemistry Solubility Rules Printable Solubility Rules Which ions are soluble Which ions are slightly soluble Which ions are insoluble How to Use Solubility Rules Identify the compound whose solubility you want to check It can be helpful to write out the empirical formula so you can identify the ions that make up the compound Look up each ion in the solubility rules
Solubility Rules A solute is considered soluble if an appreciable amount of it can be dissolved in a given amount of the solvent For example both table salt NaCl and table sugar C 11 H 22 O 11 are soluble substances in water A solute is considered insoluble if very little of it dissolves in a given amount of the solvent The solubility rules say that all ionic sodium compounds are soluble and all ionic chloride compounds are soluble except for Ag Hg 2 2 and Pb 2 which are not being considered here Therefore Na 2 SO 4 and SrCl 2 are both soluble
Chemistry Solubility Rules Printable
 Chemistry Solubility Rules Printable
Chemistry Solubility Rules Printable
https://image2.slideserve.com/5256674/practice-questions-l.jpg
Insoluble less than 1 gram dissolves in a liter Slightly soluble 1 10 grams dissolves in a liter Sparingly soluble 10 30 grams dissolves in a liter Soluble more than 30 grams dissolve in a liter Precipitate what comes out of solution when a
Templates are pre-designed files or files that can be used for various purposes. They can save effort and time by offering a ready-made format and design for developing various kinds of content. Templates can be used for individual or professional projects, such as resumes, invites, flyers, newsletters, reports, presentations, and more.
Chemistry Solubility Rules Printable

6 Fresh Solubility And Concentration Worksheet Mate Template Design

Solubility Rules Flowchart Chart Chemistry

Solubility Rules Flowchart Chart Chemistry

Chemistry Solubility Rules Free Images At Clker Vector Clip Art
Solubility Rules Zumdahl

15 Ion Worksheet High School Worksheeto

https://chem.libretexts.org//Equilibria/Solubilty/Solubility_Rules
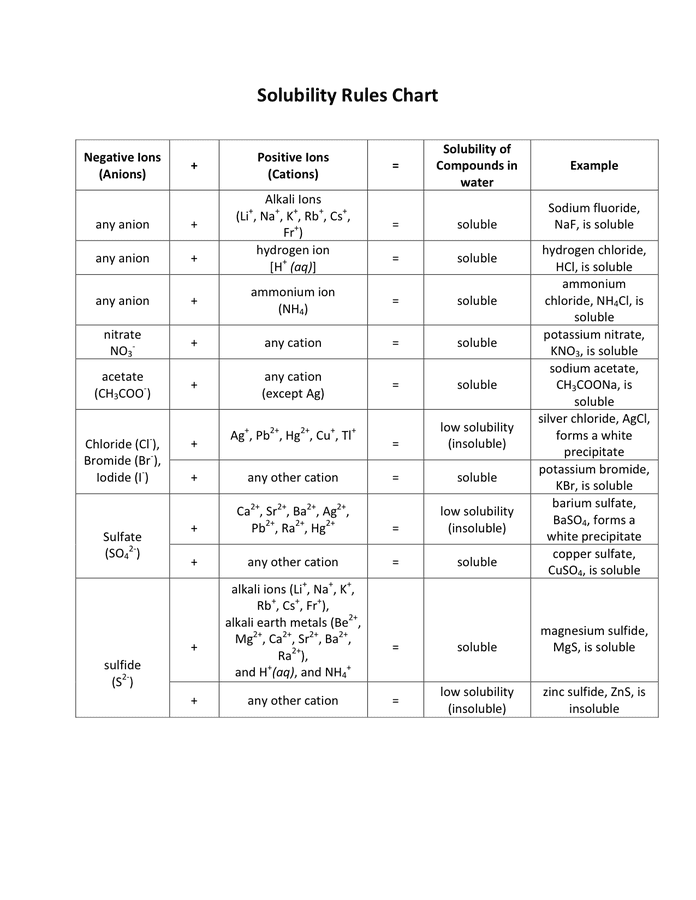
Solubility Rules The following are the solubility rules for common ionic solids If there two rules appear to contradict each other the preceding rule takes precedence Salts containing Group I elements Li Na K Cs Rb are soluble There are few exceptions to this rule Salts containing the ammonium ion NH 4 are
https://staff.concord.org//handouts/Solubility_Rules_and_ion…
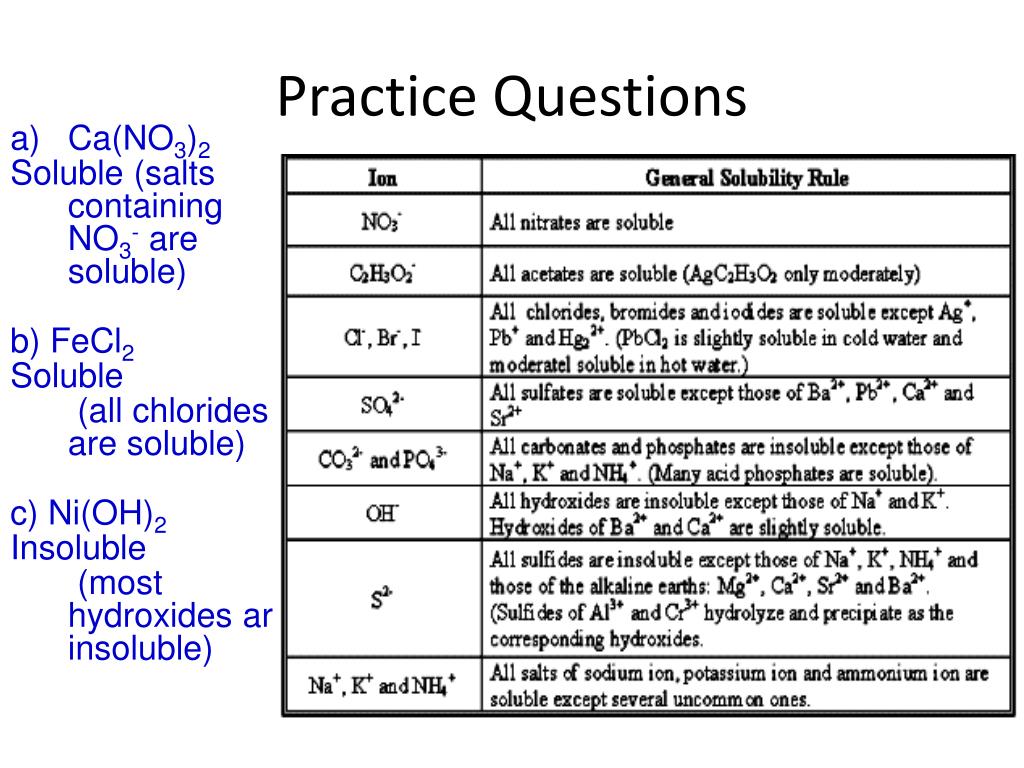
Solubility Rules NO3 1 All nitrates are soluble Cl 1 All chlorides are soluble except AgCl PbCl2 and Hg2Cl2 SO4 2 All sulfates are soluble except Ag2SO4 PbSO4 Hg2SO4 CaSO4 SrSO4 and BaSO4 CO3 2 OH 1 S 2 The carbonate of Group 1 metals and NH4 2CO3 are soluble All other carbonates are insoluble

https://www.sigmaaldrich.com/US/en/technical-documents/technical
Limescale or soap scum are precipitates left behind when water with higher mineral content evaporates and introduces previously dissolved metal cations to carbonates or soap anions Solubility is applicable to many laboratory processes and is also important in medicine

https://blog.prepscholar.com/solubility-rules-chart-chemistry
A solute is considered insoluble when they are unable to dissolve at a ratio greater than 10000 1 While many compounds are partially or mostly insoluble there is no substance that is completely insoluble in water meaning that it can t dissolve at all

https://getchemistryhelp.com//uploads/2013/05/Solubility-Ru…
Solubility Rules Use the following rules to determine if an ionic compound will be soluble aq or insoluble s For more practice problems and video lessons visit GetChemistryHelp
SOLUBILITY RULES 1 Salts of ammonium NH 4 and Group IA are always soluble 2 a All chlorides Cl are soluble except AgCl Hg 2 Cl 2 and PbCl 2 which are insoluble b All bromides Br are soluble except AgBr Hg 2 Br 2 HgBr 2 and PbBr 2 which are insoluble c All iodides I are soluble except AgI Hg 2 I 2 HgI 2 and PbI 2 The Organic Chemistry Tutor 268 09 31 Solubility Rules Explanation Practice Wayne Breslyn 301 Mr Causey 191 07 35 Solubility Rules and How to Use a Solubility Table Melissa Maribel 502 02 41 Solubility Rules Mnemonic Tricks Club Academia 458 Showing 1 of 14 videos Load more videos Click to get Pearson app Download
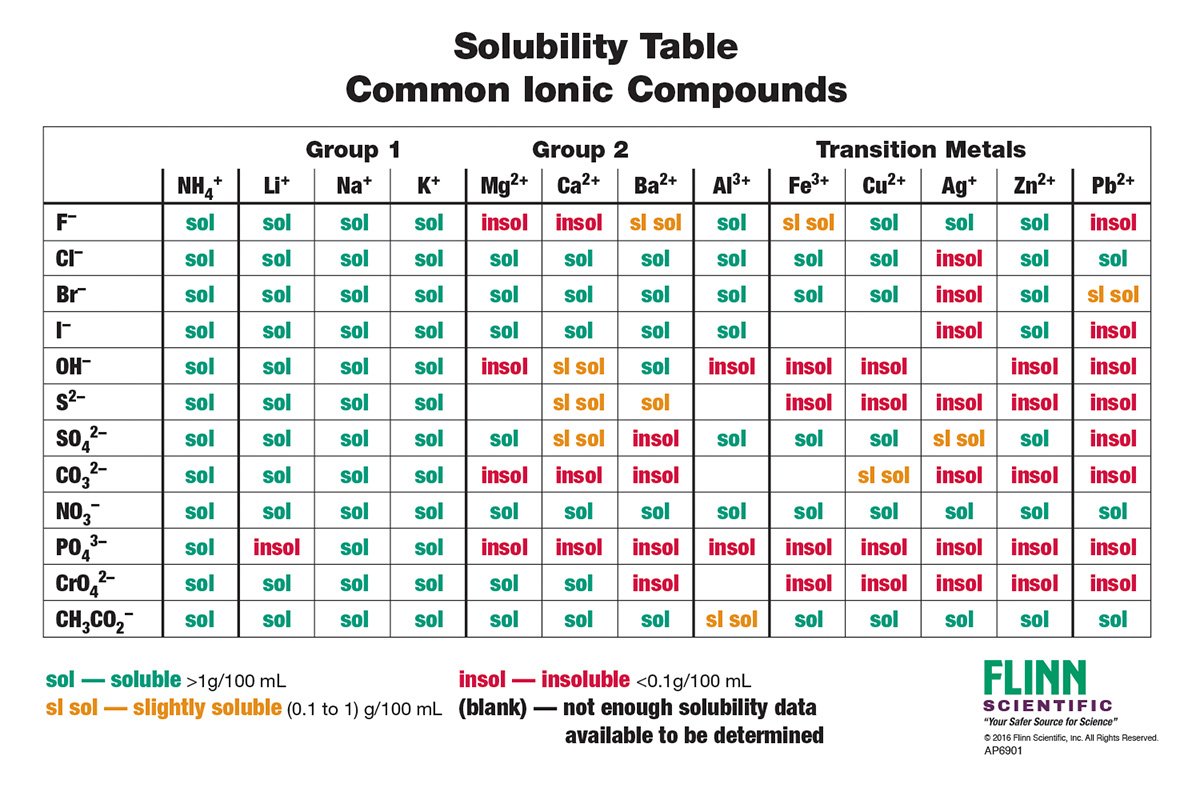
With over 140 compounds listed this comprehensive chart clearly shows the solubility trends or patterns for ionic compounds and helps reinforce general rules for determining solubility The table is arranged with groups of cations listed across the top and anions listed on the side