Activity Series Of Metals Printable Activity Series of Metals The Activity Series of the metals is a n invaluable aid to predicting the products of replacement reactions It also can be used as an aid in predicting products of some other reactions Pay attention to the notes below as they are provided to help you make better use of the activity series tha n just the list of
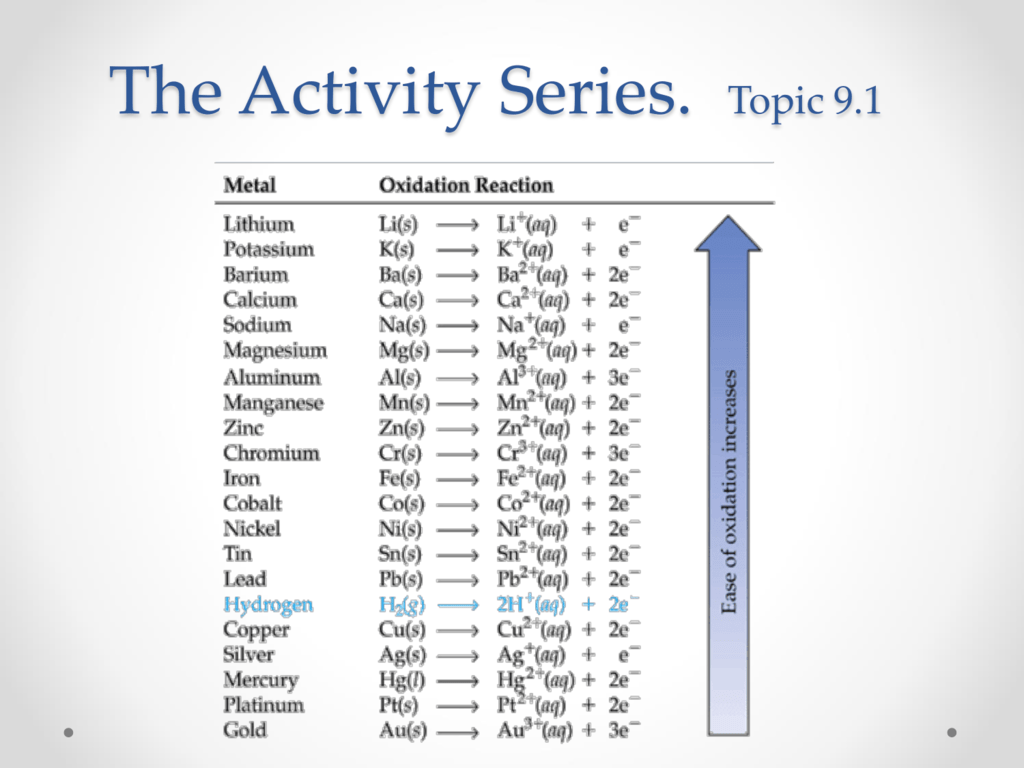
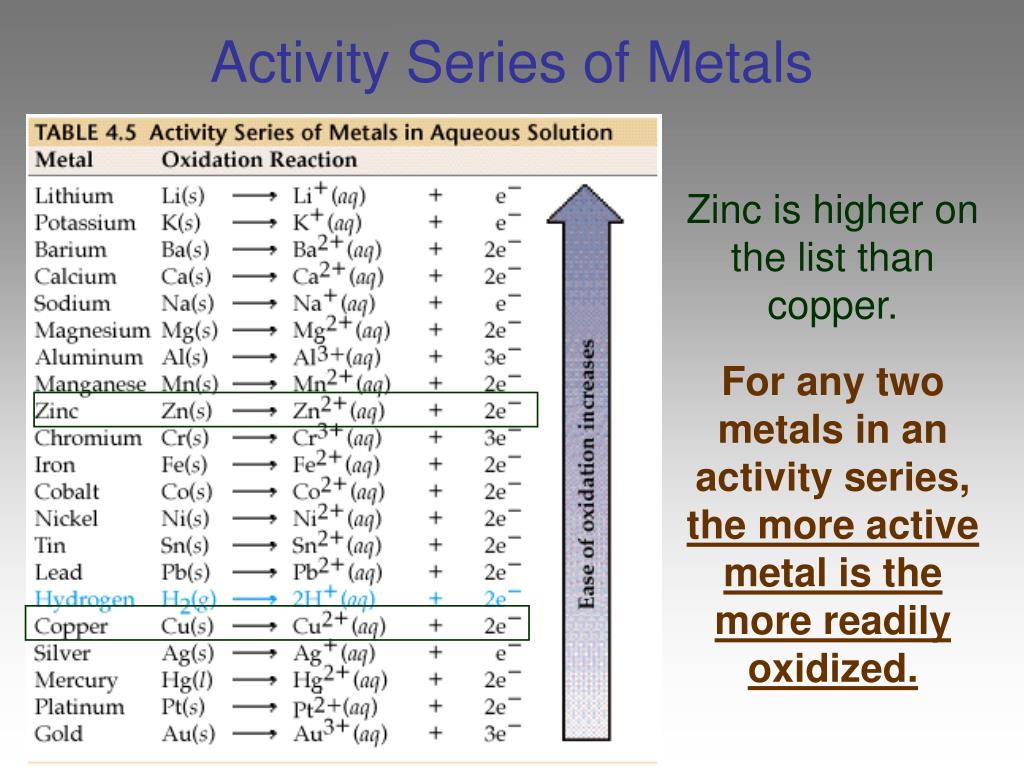
The activity series is a chart of metals listed in order of declining relative reactivity The top metals are more reactive than the metals on the bottom For example both magnesium and zinc can react with hydrogen ions to displace H The activity series is a list of elements in decreasing order of their reactivity Since metals replace other metals while nonmetals replace other nonmetals they each have a separate activity series The table below is an activity series of
Activity Series Of Metals Printable
 Activity Series Of Metals Printable
Activity Series Of Metals Printable
https://s3.studylib.net/store/data/009281468_1-8e85b621d4ca7b02a9a0a535e951ecdc.png
Metals are usually reclaimed from their ores in one of two ways chemical reduction using carbon in the form of coke or electrolytic reduction using electricity Carbon is a relatively cheap way to reduce metals from their ores Carbon fits on the activity series scale between aluminum and zinc you can assign it a rating of 8
Pre-crafted templates offer a time-saving solution for creating a varied series of documents and files. These pre-designed formats and designs can be used for different individual and expert tasks, including resumes, invites, leaflets, newsletters, reports, discussions, and more, improving the material creation procedure.
Activity Series Of Metals Printable

PPT Why Do Chemists Like NO 3 s So Much PowerPoint Presentation
Activity Series Of Metals Complete You Can Continuation Recite Posts

PPT Electrochemistry PowerPoint Presentation Free Download ID 3527121

Solved Use The Activity Series metals Table To Determine If Chegg
PPT Chapter 21 Electrochemistry PowerPoint Presentation Free
Amphoteric Oxides Reactivity Series Or Activity Series Of Metals
https://chem.libretexts.org/Ancillary_Materials/Worksheets/Worksheets
Activity Series of Metals In bioinorganic and inorganic reactions there are only a few metals that are really useful for redox Chemists are interested in trying to find the best metals to utilize Activity is the tendency of a metal and hydrogen to LOSE electrons M s Mn ne Is this an oxidation or a reduction Explain

https://chemdemos.uoregon.edu/sites/chemdemos1.uoregon.ed…
Based on their knowledge of the uses of metals and then design a guided inquiry experiment to rank metals based on their pair wise reactions with metal ions For best results we suggest a minimum of six combinations of metals and metal ions A great critical thinking question to cap the exercise is If you do the corresponding reactions of
.PNG?w=186)
https://chemguide.net/wp-content/uploads/2017/11/Activity-Ser…
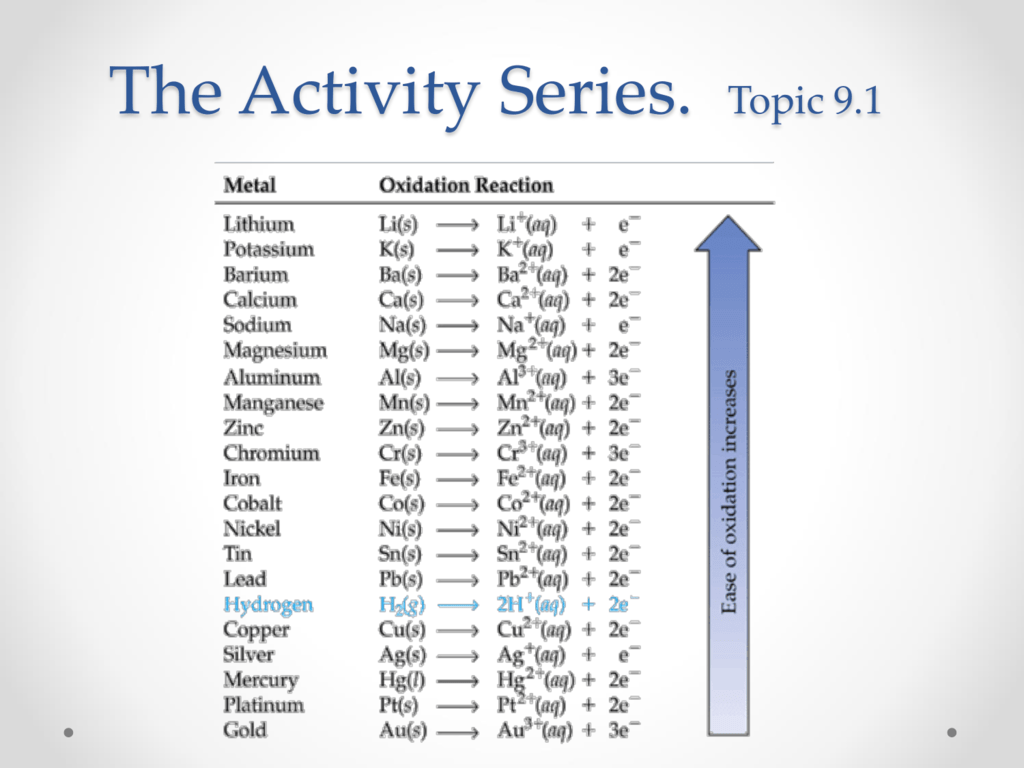
An activity series is a list of metals arranged in order of decreasing ease of oxidation The metals at the top of the table especially the alkali and alkali earth metals are the most easily oxidized They are called active metals

https://sites.prairiesouth.ca//tables_charts/activity_series.pdf
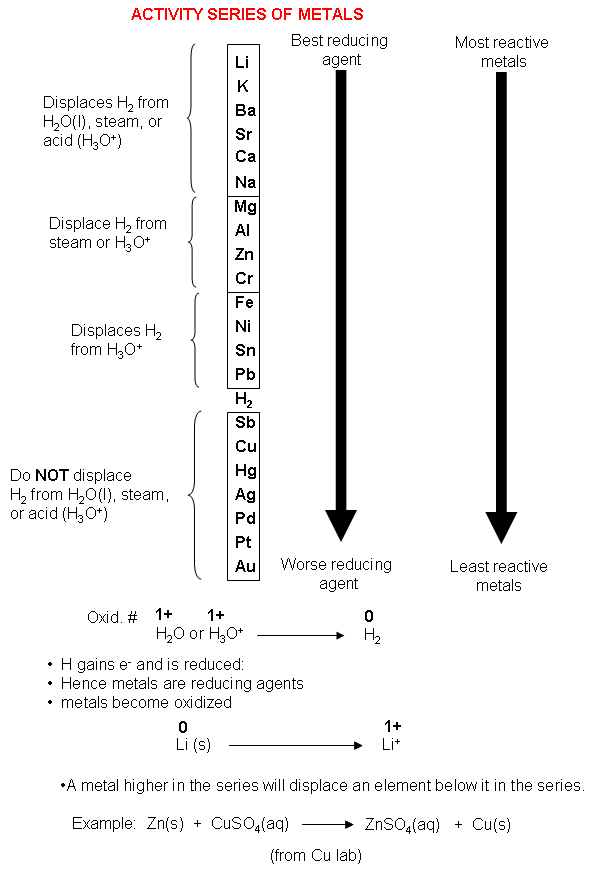
These metals displace hydrogen from acids Zn s HCl aq ZnCl2 H2 g hydrogen H copper Cu silver Ag mercury Hg platinum Pt gold Au These metals do not displace hydrogen from acids or water These elements are more stable and form compounds less readily than do those found higher in the table least active

https://sciencenotes.org/activity-series-of-metals-reactivity-series
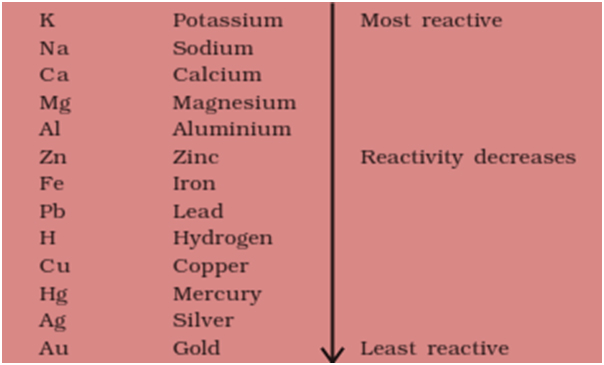
The activity series of metals or reactivity series is a list of metals from most reactive to least reactive Knowing the activity series helps you predict whether or not a chemical reaction occurs Specifically use it for identifying whether a metal reacts with water or acid or whether it replaces another metal in a reaction
Ca s 2H 2O Ca OH l 2 aq H 2 g Cu s HOH l NR The Activity Series is NOT just metals Some halogens will displace other halogens in compounds Cl2 2NaI I2 NaCl I NaCl NR 2 No reaction NaCl aq F2 g NaF Cl2 2 Mg s LiOH aq No Reaction The Activity Series of Metals Handout The activity series is a series of metals in order of reactivity from highest to lowest It is used to determine the products of single displacement reactions whereby metal A will replace another metal B in a solution if A is higher in the series Activity series of some of the more common metals listed in
Chrome reader mode Enter Reader Mode